A number of common diseases, neurodegenerative disorders such as Alzheimer's disease (AD), Parkinson's disease (PD), Huntington disease (HD), transmissible spongiform encephalopathy (TSE) and amyotrophic lateral sclerosis (ALS), as well as metabolic disorders such as familial transthyretin amyloidosis (FTA) and type 2 diabetes (T2D), are associated with defective protein turnover. The hallmark of these diseases, belonging to the broad class of proteopathy diseases, is misfolding, aggregation and tissue-specific accumulation of aggregated protein deposits composed of specific peptides/proteins, such as: amyloid-beta (A-beta) and tau in AD, alpha-synuclein in PD, poly-Q extended huntingtin in HD, prion protein (PrP) in TSE, superoxide dismutase 1 (SOD1) in ALS, transthyretin (TTR) in FTA, and islet amyloid polypeptide (IAPP) in T2D. While it is not fully understood why these otherwise soluble and flexible peptide/protein molecules begin to self-assemble and build structured aggregates; it is well established that the formation and accumulation of structured peptide/protein aggregates is an early sign of imbalance in peptide/protein physiological turnover, i.e. an early sign of disease. The possibility to detect the first (timely) appearance of abnormally increased levels of structured peptide/protein aggregates in the peripheral blood circulation (other biological fluids) and to monitor their change over time under pharmacotherapy with disease modifying drugs, presents a unique opportunity for early diagnosis of proteopathy diseases and for personalized approaches to their treatment.
The all-encompassing goal of this research project is to develop specialized photonics instrumentation and mathematical analysis tools that will allow us to translate our innovative method for ultrasensitive detection of structured peptide/protein aggregates to the primary care and use it to diagnose devastating proteopathy diseases with the ultimate, single-molecule sensitivity.
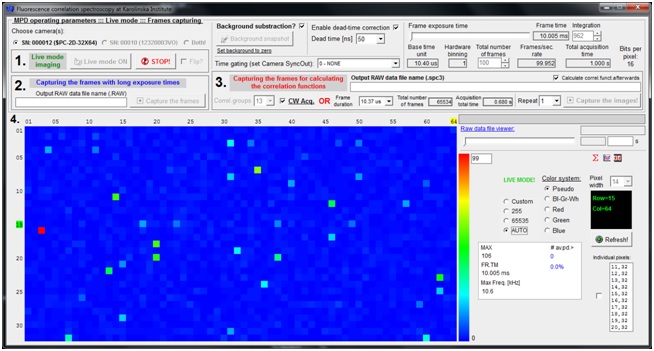
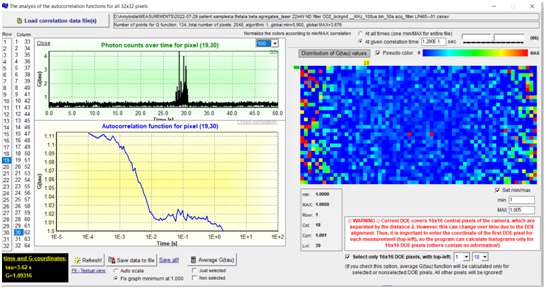
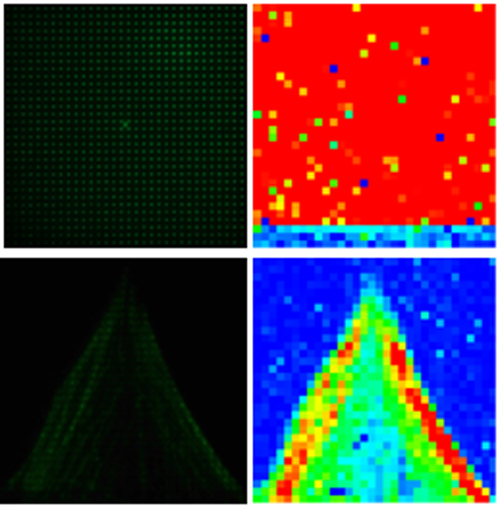
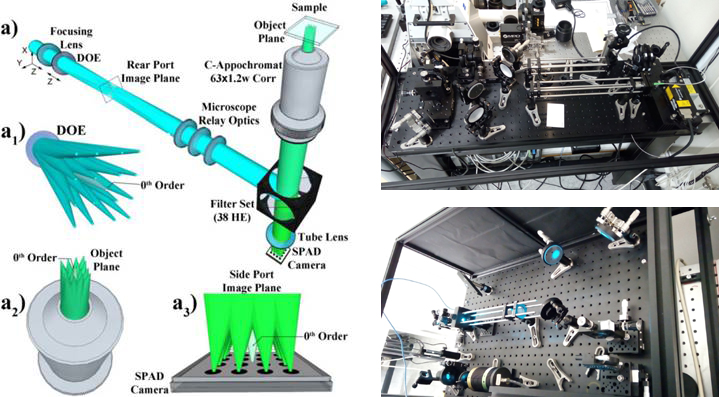
To achieve this ambitious goal, an interdisciplinary team of specialists from TAMUQ and KI, with diverse yet complementary expertise in biomedicine, physical chemistry, photonics and applied mathematics, will work together on the following specific aims. WP1 will develop a compact, table-top instrument for massively parallel Fluorescence Correlation Spectroscopy (mpFCS) that will allow medical professionals to identify by screening individuals with elevated levels of structured peptide/protein aggregates in the blood serum, who are at risk to develop/have developed a proteopathy disease. WP2 will develop a compact, table-top instrument for massively parallel dual color Fluorescence Cross-Correlation Spectroscopy (mpFCCS) that will allow medical professionals to specifically diagnose the proteopathy disease in individuals who are identified by screening to have elevated levels of structured protein aggregates. WP3 will develop new software tools for fast acquisition, analysis and representation of large data sets.
This project is of great general bearing and with a strong innovative and commercial potential. Most notably, this project holds the promise to develop a new methodology for early, rapid and cost-effective diagnosis of proteopathy diseases, for some of which, such as AD, there is presently no efficient early diagnosis and, as a consequence, also no cure. Moreover, this minimally invasive and cost-effective method allows continuous monitoring of the effect of disease-modifying drugs on disease progression, thus allowing the physician to develop personalized treatment strategies. Finally, our method holds the possibility to evaluate with unprecedented sensitivity the capacity of selected agents to modify peptide/protein aggregation. This, together with the possibility to identify patients at a very early disease stage, when the underlying physiological balance is disturbed but the degenerative effects are small and not yet noticeable at the organism level, is of utmost importance and will significantly aid drug discovery.
In addition to applications in healthcare, the methodology and instrumentation developed in this project are of direct relevance for the pharmaceutic and cosmetic industries for the quality control of peptide/protein therapeutics, e.g. insulin, and peptide/protein-based skin- and hair-care formulations. This is of utmost importance since the spontaneous formation of structured aggregates may significantly reduce the efficacy of peptide/protein therapeutics and/or cause adverse effects, such as allergies.
Furthermore, the instrumentation and data acquisition, together with large data-sets sampling and processing routines that will be developed, are invaluable for biomedical research as they enable us to quantitatively characterize fast dynamical processes and map in live cells the dynamic reaction-diffusion landscape.